By: Rebecca J. Safran ( Department of Ecology and Evolutionary Biology University of Colorado, Boulder ) & Patrik Nosil ( Department of Ecology and Evolutionary Biology University of Colorado, Boulder ) © 2012 Nature Education
![]()
Citation: Safran, R. J. & Nosil, P. (2012) Speciation: The Origin of New Species. Nature Education Knowledge 3( 10 ) :17
![]()


![]()
![]()
How do new species form? Like most areas of Evolutionary Biology, research related to the formation of new species - 'speciation ' - is rich in historical and current debate. Here, we review both early and modern views on speciation, starting with Darwin and finishing with current genomics-era insights.
". these forms may still be only . varieties; but we have only to suppose the steps of modification to be more numerous or greater in amount, to convert these forms into species . thus species are multiplied" (Darwin 1859, p. 120).
Discussion of most topics within Evolutionary Biology begins with Darwin. Indeed, On The Origin of Species (1859) continues to influence much of modern Evolutionary Biology. Darwin viewed evolution by natural selection as a very gradual mechanism of change within populations, and postulated that new species could be the product of this very same process, but over even longer periods of time. This eventual process of speciation by natural selection is illustrated by a sketch drawn by Darwin in his personal notebook nearly 20 years before the Origin of Species was published (Figure 1). Here, he proposed a model whereby lineages form from their ancestors by evolving different characters over relatively long periods of time. Darwin indicated that species could form by the evolution of one species splitting into two, or via a population diverging from its extant ancestor to the point it was a new species. Darwin's insights into evolution were brilliant, especially in light of their being made in the absence of genetics. Indeed, ideas about heredity and the introduction of new genetic material via mutation were to come long after Darwin's founding theories of evolution.
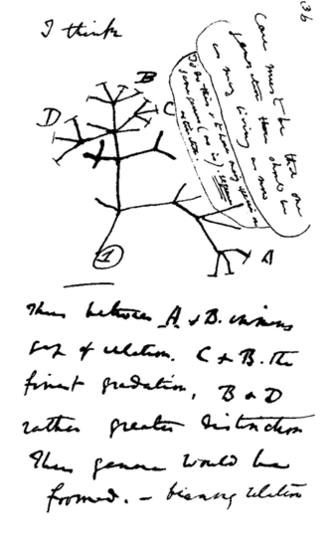
Figure 1: Darwin’s famous sketch indicating that evolution within species may eventually give rise to entirely new ones.
![]()
Image via Wikimedia Commons. Some rights reserved.
A major turning point for evolutionary research occurred in the 1930s when Fisher, Haldane, Wright, Dobzhansky, and others, developed mathematical population genetic models to illuminate the genetic mechanisms of evolutionary change (Mayr & Provine 1998). The integration of genetics with models of natural selection shed tremendous light on, and strengthened Darwin's views on, evolution — here was the missing mechanism that introduced new variation into populations via mutation and recombination. Indeed, thanks to the Modern Synthesis, much of current research in Evolutionary Biology is strongly tied to genetics, and current methods for studying speciation are no exception. As discussed below, the Modern Synthesis led to advances not only in the study of evolution within populations, but also changes in the way species were defined, and in how new species were considered to form.
Under the commonly used ‘Biological Species Concept' (Mayr 1942), the formation of new species involves the evolution of reproductive barriers to the production of viable offspring either before (pre-zygotic barriers) or soon after (post-zygotic barriers) mating. Thus, new species form when individuals from diverging populations no longer recognize one another as potential mates, or opportunities for mating become limited by differences in habitat use or reproductive schedules. In some cases, these pre-zygotic isolating mechanisms fail to prevent inter-breeding among individuals from separate populations. In these cases, viable hybrids may form, or the consequences of a successful mating attempt may end in failure, either due to the production of inviable zygotes or sterile, non-reproductive offspring. These diverse pre- and post-zygotic barriers are of great importance to speciation biologists because they determine how reproductively-isolated populations are from one another, which indicates how far along the often continuous process of speciation that populations are. For example, reproductive isolation is weak in the early stages of speciation, but changes to strong or complete in later stages of speciation (Figure 2). One or more of the many types of isolating mechanisms may play a role in the evolution of species along a continuum (Figure 2). But how and why might reproductive barriers to genetic exchange evolve?
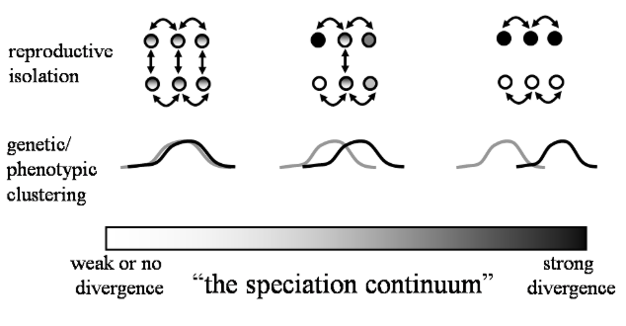
Figure 2: Schematic illustration of the continuous nature of divergence during speciation, with three arbitrary points along the speciation continuum depicted.
Numerous types of differentiation can vary quantitatively, with the magnitude of differentiation representing a measure of how far speciation has proceeded. Two headed arrows represent mating between individuals.
![]()
© 2012 Nature Education Modified and reprinted with permission from Nosil et al. 2009. All rights reserved.
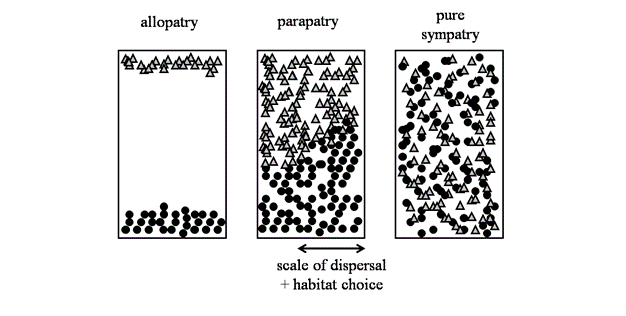
A major area of debate among speciation biologists is the geographic context in which it occurs (Figure 3). Ernst Mayr emphatically defended his view that speciation was most likely when populations became geographically isolated from one another, such that evolution within isolated populations would lead to enough differences among them that speciation would be an eventual outcome. "The . evolution of isolating mechanisms as a by-product of the steady genetic divergence is inevitable" (Mayr 1963, p. 581). The central idea here is that when populations are geographically separated, they will diverge from one another, both in the way they look and genetically. These changes might occur by natural selection or by random chance (i.e., genetic drift), and in both cases result in reproductive isolation. This view of speciation of geographically isolated populations — termed allopatric speciation — is still widely held among speciation biologists as playing a major role in the evolution of biodiversity (e.g., Price 2007).However, speciation might also occur in overlapping populations that are not geographically isolated (i.e., sympatric speciation, Via 2001). The problem here is how do populations that are living in the same area, and exchanging genes, diverge from one another? Many biologists think this will be extremely difficult (Coyne & Orr 2004), but there are a few compelling examples where populations in different habitats are subject to contrasting patterns of natural selection (i.e., divergent selection) and overcome gene flow to diverge into different species. This could occur, for example, if insects adapted to living on different plants within the same geographic region (Feder et al. 1988). It will be interesting to see how many new examples emerge now that the idea of sympatric speciation is becoming less controversial. Another scenario for speciation in the face of gene flow, albeit at levels that are lower than during sympatric speciation, is ‘parapatric speciation'. Parapatric speciation refers to populations that are situated in geographic proximity to one another, usually with abutting but non-overlapping ranges. Here, a small proportion of each population are in actual contact with one another, and thus considered in sympatry, whereas the majority of individuals reside far enough apart that frequent encounters with one another are rare (Figure 3). There are putative examples of parapatric speciation in salamanders (Niemiller et al. 2008) and walking-stick insects (Nosil et al. 2002), but the phenomenon has received less attention that allopatric or sympatric speciation (Coyne & Orr 2004).
![]()
© 2012 Nature Education Reprinted with permission from Mallet et al. 2009. All rights reserved.
The 1990s saw a reclassification of modes of speciation away from schemes that focus solely on the geographic mode of divergence and towards a focus on the evolutionary process driving genetic divergence (i.e., the ‘mechanisms' of speciation). This reclassification was motivated — at least in part — by renewed interest in the extent to which the evolutionary processes which cause adaptation within species also tend to create new species. Further, although the geographic mode of divergence has important implications for speciation via patterns of gene flow and sources of selection, speciation research has reached the point where we can directly test the role of different evolutionary process in driving speciation (Butlin et al. 2008). We outline several processes that can drive speciation.
Biologists have long been fascinated with — and sought to explain — the origin and maintenance of biological diversity within and among species. Natural selection is generally recognized as a central mechanism of evolutionary change within species. Thus, natural selection plays a major role in generating the array of phenotypic and genetic diversity observed in nature. But to what extent is selection also responsible for the formation of new species (i.e., speciation)? To what extent do phenotypic and species diversity arise via the same processes, as proposed by Darwin?
Recent years have seen renewed efforts to address these questions. For example, populations living in different ecological environments (e.g., desert versus forest habitats) might undergo divergent and adaptive evolutionary change via divergent natural selection. These same evolutionary changes can also result in the populations evolving into separate species. For example, adaptation to different environments might cause differences between populations in the way individuals tend to look, smell, and behave. In turn, these differences might cause individuals from different populations to avoid mating with one another, or hybrids exhibit reduced fitness if mating occurs. Thus, the populations cease exchanging genes, thereby diverging into separate species because of the adaptive changes that occurred via natural selection. This is a simple description of the ‘ecological speciation' hypothesis (Rundle & Nosil 2005, Schluter 2009).
More specifically, ecological speciation is defined as the process by which barriers to gene flow evolve between populations as a result of ecologically-based divergent selection between environments. This process makes some simple predictions. For example, ecologically-divergent pairs of populations should exhibit greater reproductive isolation than ecologically-similar pairs of populations of similar age (Funk 1998). Figure 4 illustrates an example that supports this prediction. Other predictions are that traits involved in divergent adaptation will also cause reproductive isolation, and that levels of gene flow in nature will decrease as ecological differences between populations increase. All these predictions have now seen support, and outstanding questions concern the genetic bases of ecological speciation and the reasons why the process varies in how far it proceeds (Rundle & Nosil 2005).
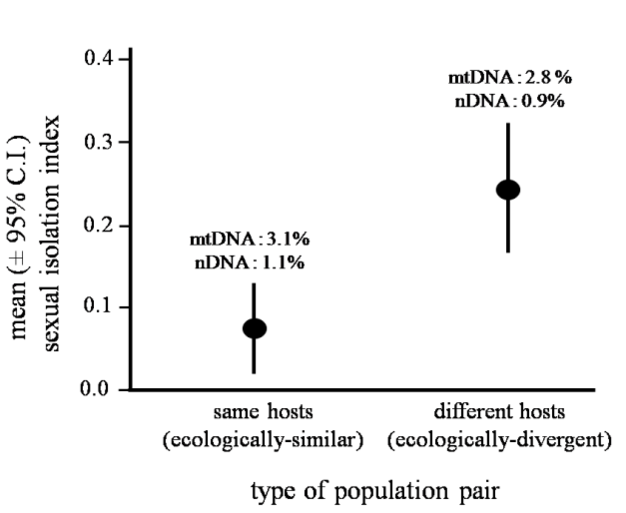
Ecological speciation in host-plant associated populations of Timema cristinae walking-stick insects (individual populations feed on either the Ceanothus spinosus host plant or on Adenostoma fasciculatum). Pairs of populations feeding on the same host plant species, but in different geographic localities, are ecologically similar and assumed to not be subject to divergent selection. In contrast, pairs of populations feeding on different host plant species are ecologically divergent and subject to divergent selection. Different-host pairs (n = 15 pairs) exhibit significantly greater reproductive isolation due to divergent mating preferences (i.e., sexual isolation) than do same-host pairs (n = 13 pairs). This pattern is independent from neutral genetic divergence, a proxy for time since divergence. Mean divergence is shown for the mitochondrial COI gene (mtDNA) and for the nuclear IT-2 gene (nDNA).
![]()
© 2012 Nature Education Modified and reprinted with permission from Nosil et al. 2002. All rights reserved.